AQS Bio
Asia Life Sciences Market Entry Solutions
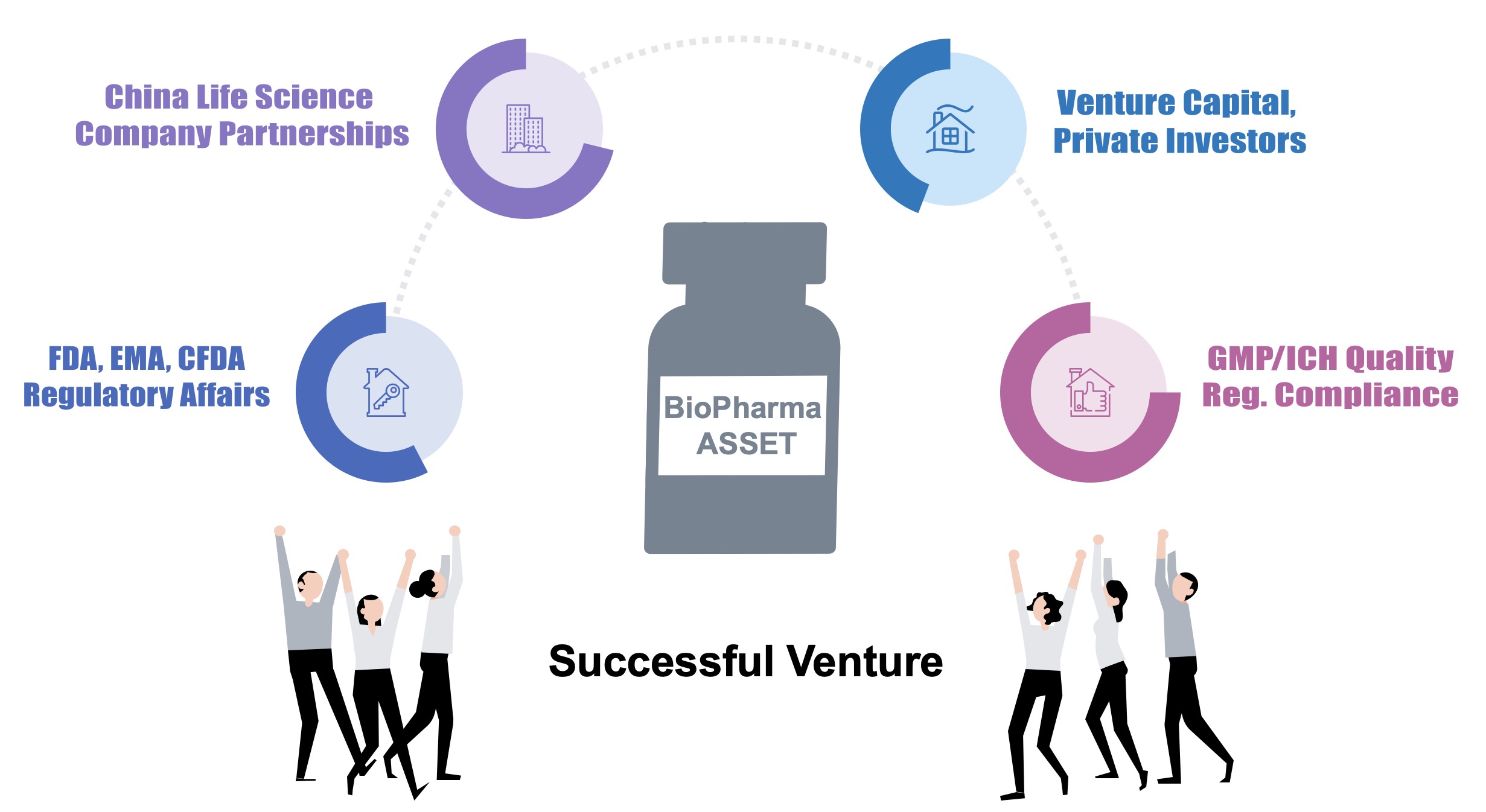
AQS Bio – an R&D biopharmaceutical company leveraging its unique experience in this market to assist other companies with out-licensing biopharma assets into China/Asia and beyond.

About

Founded by U.S. biopharma R&D, Quality, and Compliance Scientists & Engineers, alongside experienced business professionals. Successfully operating in China/Asia for over 15 years and based in Hong Kong.
Providing business development/licensing, regulatory compliance, partner search, auditing, project management, validation, regulatory submissions, and scientific consulting services for the life-science industries – reducing both risk and time to market for companies looking to out-license assets to Chinese/Asian companies. AQS Bio possesses the expertise to address any related gaps, with proven access to superior commercialization capabilities, both in China and globally.
The AQS Bio Advantage
Unparalleled China Business Development and Market Entry Capabilities.
Development
In-licensing/business development of biopharmaceutical assets for clinical/commercial development, with CMO/private investor/large China biopharma companies.
Regulatory Compliance
The ability to bring an existing lab or factory (with proven successful case) into international compliance (GLP/GMP), for biopharma clinical & commercial manufacturing.
Commercialization
Strong market and technical knowledge of the China Life sciences market from many years of auditing Chinese companies. Ability to perform regulatory submissions in China and internationally.
Marketing
Provision of business development and marketing services and consultancy.
All of these commercialization abilities equates to a decrease in time, and risk, for biopharma assets to enter the China market
Lower Risk and Faster Time to China/Global Market
AQS Bio has a wide range of China biopharma customers and contacts who are looking for advanced biopharma assets to add to, or to transition their existing product portfolios.
AQS Bio has the expertise to evaluate biopharma assets, for the China market, as well as for prospect licensee biopharma companies.
After assessing an asset’s suitability, there are options to perform clinical development/trials with a Top/Mid Tier China biopharma partner company, overseen and supported by the AQS Bio commercialization team.


China/Asia Pacific Presence
- Successful CGMP/GLP projects in China for over 15 years.
- Providing full scope of services needed for new market entry.
- Close relationship with FDA, NMPA, and EMA.
- Multi-National, Chinese Biopharmaceutical and CRO/CMO customers in China.
Over 15 years of successful technical experience in the China life science market, with the industry connections to assist with out-licensing biopharma assets, and with the expertise to expedite for both China/Global regulatory approval, and commercial production.
Get in Touch
Email Us
US Office
31B Mill River Dr.
Weymouth
MA 02188
USA
Hong Kong Office
Room 2101
21/F Futura Plaza
111 How Ming Street
Kwun Tong
Hong Kong